Fitosoil has developed a methodology for the determination of critical relative humidity in solid materials, a parameter of great importance in solid fertiliser products.
The critical relative humidity (CHR) of a salt is defined as the relative humidity of the surrounding atmosphere at which the material begins to absorb moisture from the atmosphere and below which it will not absorb atmospheric moisture. When the humidity of the atmosphere is equal to or greater than the critical relative humidity of a salt sample, the sample will take up water until the salt dissolves to obtain a saturated solution.
All water-soluble salts and mixtures have a characteristic critical humidity, which is a unique property of the material. The critical relative humidity of most salts decreases with increasing temperature. For example, the critical relative humidity of ammonium nitrate decreases by 22% with a temperature of 0 °C to 40 °C / 32 °F to 104 °C. The critical relative humidity of various fertiliser salts is given in table 1:
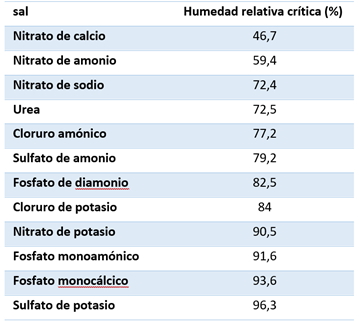
Table 1: Critical relative humidity of pure salts at 30 °C.
Mixtures of salts usually have a lower critical humidity than any of their components. Fertilisers containing urea as an ingredient typically exhibit a much lower critical relative humidity than fertilisers without urea. Table 2 shows CRH data for two-component blends:
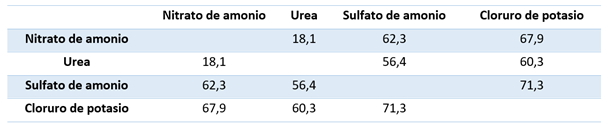
Table 2: CRH for two-component blends
The critical relative humidity of salt mixtures in the 30°C range is the percentage relative humidity. As shown, the effect of the salt mixture is most dramatic in the case of ammonium nitrate with urea. This compound has an extremely low critical relative humidity and can therefore only be used in so-called UAN solutions of liquid fertilisers.
About Fitosoil
Fitosoil is an ISO 17025-accredited private laboratory specialising in advanced analysis for the agri-food, environmental, industrial, and public health sectors. Our expert team and cutting-edge technology help our clients to comply with regulations, ensure safety, and improve their processes. As part of the Cotecna Group, a world leader in inspection, certification and analysis, we are committed to providing quality and reliable services. At Fitosoil, we provide accurate, customised analytical solutions tailored to each client's specific requirements.


